How Many Unpaired Electrons Does P Have
The external energy level partially or completely occupied by electrons is the third in fact the Mg belongs to the 3rd period. There are three unpaired electrons.
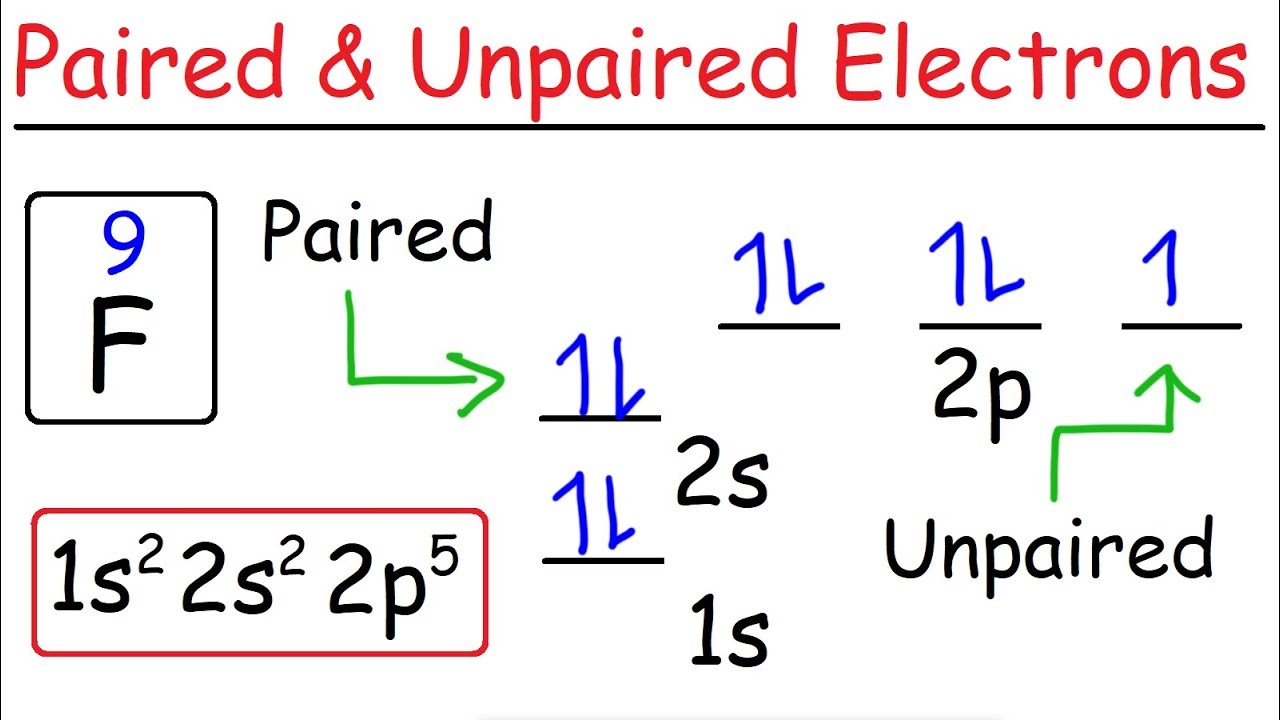
How To Determine The Number Of Paired And Unpaired Electrons Youtube
How many unpaired electrons does P have.
. Here the electron configuration shows that three unpaired electrons exist in the last orbit of phosphorus. How many unpaired electrons does MG have. Then since there is no more empty orbital at that energy level the next electron would have to pair up.
As for the number of unpaired electrons there should be 2 unpaired electrons for Mg when it is on its own. Therefore the valency of the phosphorus atom is 3. The electron configuration is.
Therefore the ground state of atomic oxygen has two unpaired electrons bi-radical and is designated as 3P triplet P state. Does P have 2 unpaired electrons. So there are 4 unpaired electrons.
The orbit filling diagram and the electron dot diagram show the empty spaces for three more electrons. 5 Can an atom have a 3d subshell. For finding the number of unpaired electrons then first we have to find the atomic number of the element then write the configuration in the ground state then according to the oxidation state subtract the number of electrons from the outer shell.
These electrons can form bonds with other elements and are called valence electrons. To determine the number of unpaired electrons draw the orbital diagram for the valence electrons. 7 What is the electron configuration of Co 3.
In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. The electron configuration of phosphorus P in excited state is P 15 1s2 2s2 2p6 3s2 3px1 3py1 3pz1. Two electrons are in the 3s orbital and the remaining three are.
8 How many electrons do Co have. NO has 15 electrons and has a bond order of 2. A molecule with one or more unpaired electrons is paramagnetic or odd electron system is paramagnetic.
Therefore there are three unpaired electrons present in. For finding the number of unpaired electrons then first we have to find the atomic number of the element then write the configuration in the ground state then according to the oxidation state subtract the number of electrons from the outer shell. Sulfur has two pairs of unpaired electrons 4 unpaired electrons.
To sum it up Silicon has two unpaired electrons. Were looking at the 3p subshell which has three orbitals. For a total of 15 electrons.
6 How many electrons does a Co atom have in its 3d subshell number of electrons 3d electrons How many of those electrons are unpaired number of unpaired electrons. In this case the. Does oxygen have unpaired electrons.
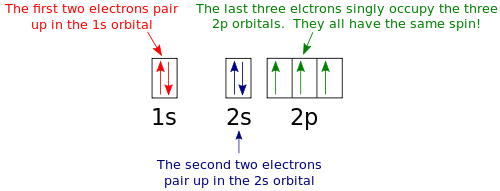
It has 2 unpaired electrons. Thus 2 electrons in 1s orbital have only paired electrons. S exclusion principle teh added second electron will be of opposite spin.
Two of the three orbitals each contain one electron. The ground state electron configuration of a phosphorous atom has three unpaired electrons. 9 What is the electron configuration of AR.
Ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p x 1 3p y 1 3p z 1. Selenium contains four unpaired electrons in its outermost orbital. Does Ni have 3 unpaired electrons.
How many electrons are unpaired in he. The nex six electrons will go in the 2p. These two electrons rotate about their own axis in parallel spins.
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. It is located in group 16 below oxygen and sulfur. So there are 3 unpaired electrons.
Were looking at the 3p subshell which has three orbitals. The very same is for 2s i beg your pardon holds 2 2p i m sorry holds 6 3s i beg your pardon holds 2. Two of the three orbitals each contain one electron.
This group of elements is sometimes called the chalcogens. How many unpaired electrons does a gaseous atom of phosphorus P have in its ground state. On the periodic table selenium is element number 34.
Each chlorine atom shares one pair of electrons with the sulfur atom resulting in two S-Cl bonds. Here this electron configuration shows that three unpaired electrons exist in the last orbit of phosphorus. There are 3 unpaired electrons in phosphorus.
And in N atoms four electrons are occupied by 1s and 2s orbitals and the remaining three are singly filled in the three orbitals present in the p subshell. So there are 4. Two unpaired electrons In ground state the oxygen molecule has two unpaired electrons each of which are located in a different pi antibonding orbital Figure 3.
5 and has 1 unpaired electron in π antibonding orbitals. In the last level of energy there are a total of two electrons in fact. So there are 3 unpaired electrons.
Phosphorus has 5 valence electrons. How many unpaired electrons does P have. So there are 4 unpaired electrons.
There are three sub-shells in the p level so the 2 electrons in the p level are free to be in different sub-shells. Phosphorus Electrons - 18 images - makethebrainhappy how many valence electrons are in an elements atoms and the periodic table is there a point phosphorus is element 15 18 8 occurrence preparation and properties of phosphorus. A 1 B 3 C 5 D 7.
The next electron would go into the last orbital. As you can see in the electron configuration the 3p sublevel has room for three more electrons. Theyre in the 3p sublevel.
10 How many electrons. Since 1s can only host 2 electrons and P has actually 15 thats obviously filled and also has no unpaired electrons. It has 2 unpaired electrons.
For finding the number of unpaired electrons then first we have to find the atomic number of the element then write the configuration in the ground state then according to the oxidation state subtract the number of electrons from the outer shell. The number of unpaired electrons in the last orbit of an element is the valency of that element.
How Are The Number Of Unpaired Electrons Determined Quora

High School Chemistry Orbital Configurations Wikibooks Open Books For An Open World

How To Determine The Number Of Paired And Unpaired Electrons Youtube
No comments for "How Many Unpaired Electrons Does P Have"
Post a Comment